
CATS
BRAVECTO FOR CATS

CATS
BRAVECTO FOR CATS
Fast, easy, lasting
flea and tick defense
Fast, easy, lasting
flea and tick defense
The first US product with protection against the Asian longhorned tick
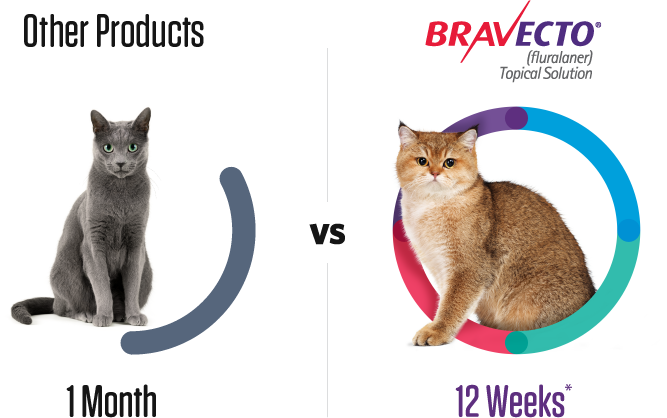
One dose of BRAVECTO protects your cat nearly 3X longer* than one dose of any monthly treatment.1-3
Available by prescription only.
The first US product with protection against the Asian longhorned tick
One dose of BRAVECTO protects your cat nearly 3X longer* than one dose of any monthly treatment.1-3
Available by prescription only.


The purrfect pick for your pets!
Longer lasting. Fewer treatments.
Less hassle for you and your cat.
Available as a topical solution with an easy
Twist’n’Use™ applicator.

The purrfect pick for
your pets!
Longer lasting. Fewer treatments. Less hassle for you and your cat.
Available as a topical solution with an easy Twist‘n‘Use™ applicator.
See the difference BRAVECTO makes for veterinarians and pet parents
“Just because your cat doesn’t go outdoors, doesn’t mean that the other tenants in your apartment building don’t have pets that go indoors and outdoors, so this is a way to protect not only your home but to protect your cat too.”
Dr. Romanie Walter
SMARTVET MOBILE VETERINARY SERVICE, ILLINOIS

See the difference BRAVECTO makes for veterinarians and pet parents
“Just because your cat doesn’t go outdoors, doesn’t mean that the other tenants in your apartment building don’t have pets that go indoors and outdoors, so this is a way to protect not only your home but to protect your cat too.”

Dr. Romanie Walter
SMARTVET MOBILE VETERINARY SERVICE, ILLINOIS

Forget to protect?
Don’t leave your pets vulnerable to fleas and ticks.

Forget to protect?
Don’t leave your pets vulnerable to fleas and ticks.

Find out where you can get your paws on BRAVECTO today!

BRAVECTO is available by prescription only.

Find out where you can get your paws on BRAVECTO today!
BRAVECTO is available by
prescription only.

*BRAVECTO (fluralaner topical solution) for Cats kills fleas, prevents flea infestations, and kills ticks (black-legged tick and Asian longhorned tick) for 12 weeks. BRAVECTO Topical Solution for Cats also kills American dog ticks for 8 weeks.
References
1. Dryden MW, Canfield MS, Bocon C, et al. Parasit Vectors. 2018;11:422. 2. BRAVECTO Topical Solution for Cats [prescribing information]. Madison, NJ: Merck Animal Health; 2016. 3. FRONTLINE Plus for Cats [prescribing information]. Duluth, GA: Merial Limited; 2013.
Important Safety Information
Important Safety Information
BRAVECTO 1-MONTH Chews: indicated for dogs 8 weeks of age and older. The most commonly reported adverse reactions include itching, diarrhea, vomiting, decreased appetite, elevated ALT, lethargy, and weight loss. BRAVECTO 1-MONTH is not effective against A. americanum in puppies less than 6 months of age. BRAVECTO Chews for Dogs: The most commonly reported adverse reactions include vomiting, lethargy, diarrhea, anorexia and pruritus. In some cases, adverse events have been reported following use in breeding females. BRAVECTO Topical Solution for Dogs: The most commonly reported adverse reactions include vomiting, hair loss, diarrhea, lethargy, decreased appetite, and moist dermatitis/rash. BRAVECTO Topical Solution for Cats: The most commonly reported adverse reactions include vomiting, itching, diarrhea, hair loss, decreased appetite, lethargy, and scabs/ulcerated lesions. BRAVECTO Topical Solution for Cats is not effective against American dog ticks beyond 8 weeks of dosing. BRAVECTO PLUS Topical Solution for Cats: The most commonly reported adverse reactions include vomiting, hair loss, itching, diarrhea, lethargy, dry skin, elevated ALT, and hypersalivation. BRAVECTO PLUS has not been shown to be effective for 2 months in kittens less than 6 months of age. Use with caution in cats that are heartworm positive. The effectiveness of BRAVECTO PLUS to prevent heartworm disease after bathing or water immersion has not been evaluated.
BRAVECTO has not been shown to be effective for 12-weeks’ duration in puppies or kittens less than 6 months of age. BRAVECTO Chews and Topical Solution for dogs is not effective against the lone star tick beyond 8 weeks of dosing. BRAVECTO Topical Solution for Dogs and Cats and BRAVECTO PLUS for cats are for topical use only. Avoid oral ingestion. The safety of BRAVECTO Topical Solution for Cats and BRAVECTO PLUS has not been established in breeding, pregnant, and lactating cats.
All BRAVECTO products contain fluralaner, which is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders. Neurologic adverse reactions have been reported in cats receiving isoxazoline class drugs, even in cats without a history of neurologic disorders. Use with caution in cats with a history of neurologic disorders.




