
NEW BRAVECTO QUANTUM FOR DOGS
365 DAYs OF PROTECTION, ALL IN ONE DOSE1
Introducing
BRAVECTO
QUANTUM


A breakthrough product in an extended-release injection
Experience the BRAVECTO difference when you give your dog year-round* flea and tick protection in a single dose.
A breakthrough product in an extended-release injection
Experience the BRAVECTO difference when you give your dog year-round* flea and tick protection in a single dose
Introducing
BRAVECTO QUANTUM


A breakthrough product in an extended-release injection
Experience the BRAVECTO difference when you give your dog year-round* flea and tick protection in a single dose.
With One Dose a Year, BRAVECTO QUANTUM Gives You:
With One Dose a Year,
BRAVECTO QUANTUM
Gives You:
- Peace of mind for 12 months*
- No risk of missing a dose
- Peace of mind for 12 months*
- No risk of missing a dose

Level Up Your Dog’s Flea and Tick Protection with BRAVECTO QUANTUM


Convenient
No monthly doses or reordering for a full year—just one quick injection at your vet’s office, and you’re all set for 4 seasons of playtime and tail-wagging confidence!

Trusted
BRAVECTO QUANTUM contains the same trusted, proven active ingredient found in all BRAVECTO products—in a new convenient form.

Innovative
The first flea and tick treatment with year-round* protection in a single injection.

Proven safety &
effectiveness
Clinically proven to be safe and effective.

Level Up Your Dog’s
Flea and Tick Protection
with BRAVECTO
QUANTUM


Convenient
No monthly doses or reordering for a full year—just one quick injection at your vet’s office, and you’re all set for 4 seasons of playtime and tail-wagging confidence!

Trusted
BRAVECTO QUANTUM contains the same trusted, proven active ingredient found in all BRAVECTO products—in a new convenient form.

Innovative
The first flea and tick treatment with year-round* protection in a single injection.

Proven safety &
effectiveness
Clinically proven to be safe and effective.

BRAVECTO QUANTUM:
Protection That Acts Fast and Lasts
Give a full year* of protection to dogs 6 months of age and older with just one dose

BRAVECTO QUANTUM:
Protection That Acts Fast and Lasts
Give a full year* of protection to dogs 6 months of age and older with just one dose
Ask Your Veterinarian for
BRAVECTO QUANTUM
at Your Next Visit
Ask Your Veterinarian for BRAVECTO
QUANTUM at Your Next Visit

FOLLOW US

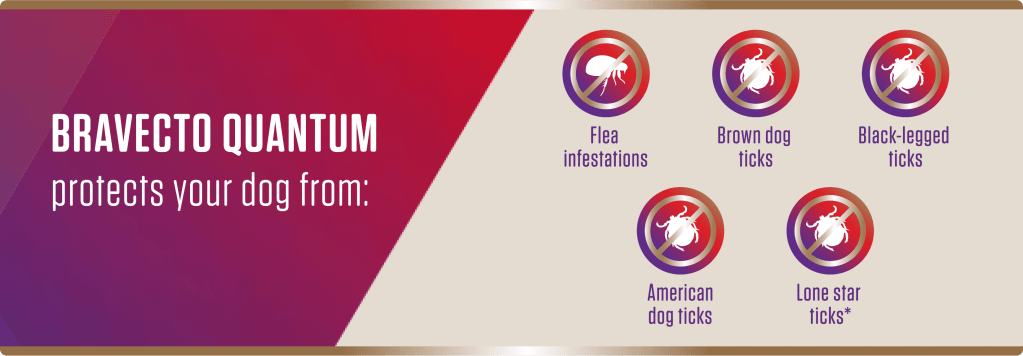
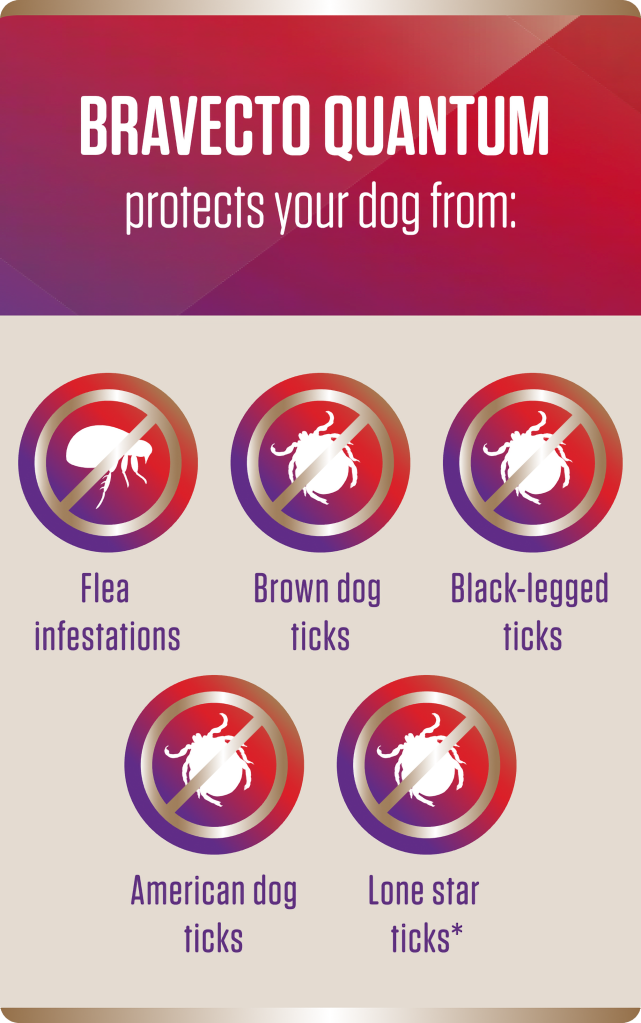
*BRAVECTO QUANTUM kills adult fleas, prevents flea infestations and treats and controls ticks (black-legged, American dog tick and brown dog tick) for 12 months. BRAVECTO QUANTUM also kills lone star ticks for 8 months.
†BRAVECTO QUANTUM does not protect against or treat vector-borne diseases.
References
1. BRAVECTO QUANTUM. Product label. Merck Animal Health; 2025. 2. Lavan R, Armstrong R, Lipworth K, Normile D, Newbury H. Flea and tick treatment satisfaction, preference, and adherence of dog owners in the United States, United Kingdom, and Australia who treated their dog with fluralaner. Open Vet J. 2020;10(2):135-143. 3. González J, Fonseca DM, Toledo A. Seasonal dynamics of tick species in the ecotone of parks and recreational areas in Middlesex County (New Jersey, USA). Insects. 2023;14(3):258. 4. McClung KL, Sundstrom KD, Lineberry MW, Grant AN, Little SE. Seasonality of Amblyomma americanum (Acari: Ixodidae) activity and prevalence of infection with tick-borne disease agents in north central Oklahoma. Vector Borne Zoonotic Dis. 2023;23(11):561-567. 5. Raghavan RK, Koestel ZL, Boorgula G, et al. Unexpected winter questing activity of ticks in the central midwestern United States. PLoS One. 2021;16(11):e0259769. 6. Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152(3-4):173-185. 7. Blagburn BL, Dryden MW. Biology, treatment, and control of flea and tick infestations. Vet Clin North Am Small Anim Pract. 2009;39(6):1173-viii.
IMPORTANT SAFETY INFORMATION
BRAVECTO (fluralaner) Chews for Dogs: The most commonly reported adverse reactions include vomiting, lethargy, diarrhea, anorexia and pruritus. In some cases, adverse events have been reported following use in breeding females. BRAVECTO Chews has not been shown to be effective for 12-weeks’ duration in puppies less than 6 months of age. BRAVECTO Chews is not effective against lone star ticks beyond 8 weeks of dosing. Indicated for dogs 6 months of age and older. BRAVECTO 1-MONTH (fluralaner) Chews: The most commonly reported adverse reactions include itching, diarrhea, vomiting, decreased appetite, elevated ALT, lethargy, and weight loss. Not effective against lone star ticks in puppies less than 6 months of age. Indicated for dogs 8 weeks of age and older. BRAVECTO (fluralaner topical solution) for Dogs: The most commonly reported adverse reactions include vomiting, hair loss, diarrhea, lethargy, decreased appetite, and moist dermatitis/rash. BRAVECTO Topical Solution for dogs has not been shown to be effective for 12-weeks’ duration in puppies less than 6 months of age. BRAVECTO Topical Solution for Dogs is not effective against lone star ticks beyond 8 weeks of dosing. For topical use only. Avoid oral ingestion. Indicated for dogs 6 months of age and older. BRAVECTO QUANTUM (fluralaner for extended-release injectable suspension) for Dogs: The most commonly reported adverse reactions in a US field study included lethargy, decreased appetite, vomiting, diarrhea, elevated liver enzymes and pruritus. BRAVECTO QUANTUM is not effective against lone star ticks beyond 8 months of dosing. Indicated for dogs 6 months of age and older.
BRAVECTO (fluralaner topical solution) for Cats: The most commonly reported adverse reactions include vomiting, itching, diarrhea, hair loss, decreased appetite, lethargy, and scabs/ulcerated lesions. BRAVECTO Topical Solution for Cats is not effective against American dog ticks beyond 8 weeks of dosing. BRAVECTO Topical Solution for Cats has not been shown to be effective for 12-weeks’ duration in kittens less than 6 months of age. The safety of BRAVECTO Topical Solution for Cats have not been established in breeding, pregnant and lactating cats. For topical use only. Avoid oral ingestion. Indicated for cats 6 months of age and older. BRAVECTO PLUS (fluralaner and moxidectin topical solution) for Cats: The most commonly reported adverse reactions include vomiting, hair loss, itching, diarrhea, lethargy, dry skin, elevated ALT, and hypersalivation. BRAVECTO PLUS has not been shown to be effective for 2 months in kittens less than 6 months of age. Use with caution in cats that are heartworm positive. The effectiveness of BRAVECTO PLUS to prevent heartworm disease after bathing or water immersion has not been evaluated. The safety of BRAVECTO PLUS have not been established in breeding, pregnant and lactating cats. Indicated for cats 6 months of age and older.
All BRAVECTO products contain fluralaner, which is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders. Neurologic adverse reactions have been reported in cats receiving isoxazoline class drugs, even in cats without a history of neurologic disorders. Use with caution in cats with a history of neurologic disorders.






